Over the last few years there have been significant advances in the management of wet age-related macular degeneration with the introduction of intravitreal vascular endothelial growth factor antibodies (anti VEGF). This treatment has revolutionised our management of wet age-related macular degeneration resulting in a significantly lower rate of visual loss than previous treatments.
The commonest drug used for injections into the eye is called Lucentis (Ranibizumab). Experience with this drug has suggested that up to 40 per cent of people with any form of wet age-related macular degeneration have shown a significant increase in their vision on treatment with the rest benefiting from a stabilisation of vision. Lucentis is a humanised anti VEGF antibody fragment that binds all VEGF isomers. Lucentis is now fully licensed in the UK and has become the standard treatment for wet age-related macular degeneration.
Below is a graph showing patients receiving Lucentis treatment compared to those who underwent either photodynamic therapy laser treatment or Sham (no treatment) group. As you can see in both studies, Lucentis patients gained significant improvements in vision.
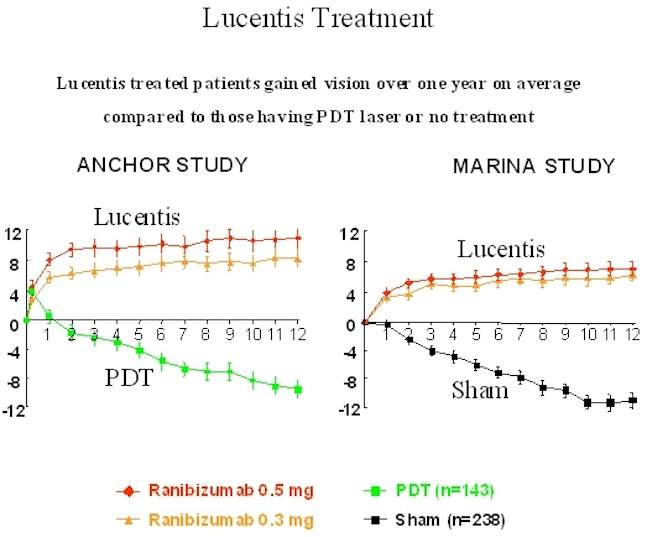
Lucentis Treatment
Lucentis is given as a series of three, monthly injections followed by review of the retina with optical coherence tomography scanning. Many patients require a further three injections due to persistent leak in the retina and some patients require many more injections over a prolonged period. Results of further trials are awaited and we are looking at additional treatments to try and stabilise the retina in those few patients who require prolonged multiple injections.
Lucentis is a fully licensed drug designed specifically for use in the eye and has now taken over from Avastin (Bevacizumab). However, Avastin remains a significantly cheaper option and is occasionally used in other conditions where Lucentis does not have a formal licence. All private health insurance companies now fully recognise anti VEGF therapy as an acceptable treatment.
Mr Tanner is now increasingly using Lucentis antigrowth factor injections for the treatment of diabetic macular oedema, retinal vein occlusion and other conditions affecting the retina where there is macular swelling
All intravitreal injections are carried out under sterile conditions to minimise the chance of any infection and will be done in a minor operating theatre environment. The injection only takes a few minutes, will be given under anaesthetic drops and is not normally painful. Following application of the drops and a small injection of anaesthetic the drug itself is given via a very small needle. The volume injected is tiny (usually 0.05ml). Patients occasionally notice floaters in the eye as the drug is injected. The procedure is carried out as a day case and you can usually leave the hospital within an hour of receiving the injection.
Over the next few days there maybe some irritation and redness of the eye but this should be mild and resolved fairly quickly. Patients may notice a few floaters in the vision but again these usually resolve fairly quickly.
If you have any problems following your injection please do not hesitate to contact my team on 0800 644 0900 or 0800 644 0700. If you have an urgent out-of-hours problem please contact the Dunedin Hospital switchboard on 0118 958 7676 or Princess Margaret Hospital Windsor on 01753 743 402 and they will be able to contact me for further advice.
Disclaimer : The information provided in this website is intended as a useful aid to general practitioners, optometrists and patients. It is impossible to diagnose and treat patients adequately without a thorough eye examination by a qualified ophthalmologist, optometrist or your general practitioner. Hopefully the information will be of use prior to and following a consultation which it supplements and does not replace.


